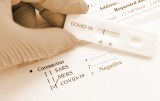
SARS-CoV-2 - Material for the development of rapid serological kits
We offer reagents for the development of COVID-19 IgG/IgM rapid tests. The kits contain essential reagents for the development of the COVID-19 IgG/IgM lateral flow test (rapid test) to aid in the qualitative detection of SARS-CoV-2 specific IgG and IgM antibodies in human biological fluids.
The rapid test is based on the Gold Immunochromatography (GICA) method for the detection of SARS-CoV-2 (2019-nCoV) specific IgG and IgM antibodies in human bilogical fluids. It consists of two test lines, one IgG and one IgM line, and a control line. The mouse monoclonal anti-human IgG antibody is pre-hybridized in the region of the IgG test line while the mouse monoclonal anti-human IgM antibody is pre-hybridized in the region of the IgM test line. Goat polyclonal anti-IgY antibody of chicken is pre-hybridized in the region of the control line. During testing, the specimen reacts with the SARS-CoV-2 (2019-nCoV) antigen-coated particles in the test cassette. The mixture then migrates upward on the membrane by capillary chromatography and reacts with human anti-IgG in the region of the IgG test line. If the specimen contains IgG antibodies to SARS-CoV-2 (2019-nCoV), a colored line will appear in the region of the IgG test line. Similarly, if the specimen contains IgM antibodies to CoV-2 SARS (2019-nCoV), a colored line will appear in the region of the IgM test line. And if the specimen tested does not contain antibodies specific for SARS-CoV-2 (2019-nCoV), no colored line will appear in either region of the test line, indicating a negative result. As a procedural control, a colored line will always appear in the control line region, indicating that the proper specimen volume has been added.
Search result : 5 product found
Refine your search :
RUOCE / IVD
- kit
Cat#
Description
Cond.
Price Bef. VAT
MBS2563898-5000
5000Tests
MBS2563898-1000
1000Tests
MBS2563898-5x1000
5x1000Tests
‹
›

