Custom Service: Monoclonal antibody library production
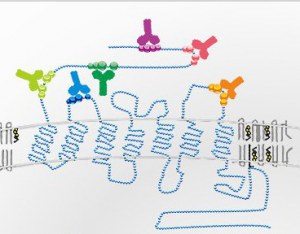
SEAL Technology for Surface Epitope Antibody Library for generating a library of high affinity monoclonal antibodies against a library of antigens representative of a protein surface.
=> Quote request
Both linear peptides and structural epitopes on the protein surface are targeted.
A typical SEAL™ library
- consists of 6-12 high affinity monoclonal antibodies
- specifcally targets 4-8 independent protein surface epitopes.
- greatly increases the chance to discover successful antibodies compared to traditional methods in which antibodies only target 1-3 epitopes against short peptides or soluble protein antigens.

Based on application results from >100 SEAL™ libraries generated for membrane proteins, soluble factors, transcription factors and kinases, on average, at least 2 times more successful antibodies for Western and IP experiments are discovered from SEAL™ antibodies than from antibodies generated by conventional methods. For difficult targets such as membrane receptors and transcription factors, SEAL™ antibodies are 3-4 times more likely to be successful. This is not surprising since the numbers of independent antibodies and epitopes targeted are multiple times larger in SEAL? antibodies and for low abundance proteins, this seems to be an even more critical factor for generating successful antibodies. SEAL? antibody library generation has significant advantages over traditional monoclonal and polyclonal antibody generation in time, cost, and most importantly, the quality of antibodies.
SEAL™ library generation
A proprietary computational model is used to identify ten-residue-long stretches that are exposed on the protein surface (SEAL™ antigens). By combining secondary structure prediction, phylogenetic analysis and structural modeling, this method strikes a 73%accuracy (67%sensitivity and 82%specificity) in identifying exposed linear peptide epitopes (SEAL™ epitopes).

Synthetic genes encoding multiple SEAL? epitopes are inserted into a proprietary DNA vector consisting of Immunogenicity Enhancement Factors (IEF, for example, multiple T epitopes) and DNA sequences that will result in particulate, highly immunogenic recombinant proteins that sample the surface of the target protein when expressed in E.Coli.

Structural domains are produced as soluble proteins in E.Coli or mammalian cells in parallel to linear SEAL™ epitopes. A proprietary bacterial expression/secretion system is used to produce soluble proteins in the culture medium.
Mice immunized with structural immunogens will produce antibodies that may recognize a few dominant conformational epitopes of the target proteins. This type of antibody is often more successful for applications such as IP, flow cytometry or functional studies.

Mice immunized with IEF-SEAL™ epitope antigens using a proprietary, optimized immunization with only a small quantity of antigens produce high affinity, IgG immune responses within three weeks. Spleen cells of the sacrificed mice arefused with SP2/0 cells and screened with quantitative competitive ELISA to select hybridoma cell lines that secrete antibodies that specifically recognize epitope sequences with at least nano molar affinity. Selected hybridomas are then subjected to multiple rounds of sub-cloning to establish stable cell lines.

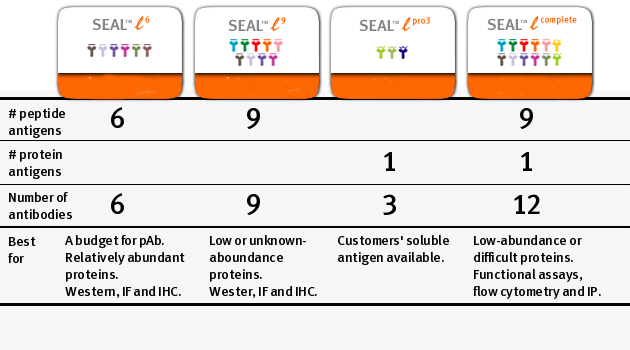
SEAL™ monoclonal antibody library products are designed to maximize success rate in developing working antibodies for diverse applications, and 4 standard type of SEAL libraries are offered to achieve this. These products differ in:
- the number and type (peptides or proteins) of antigens used for immunization
- the number of epitopes targeted
- the number of antibodies delivered to customers
The product comparison and a few useful selection rules are listed below: